Written by: Spencer Gaut
Edited by: Ryan Lee
Edited by: Ryan Lee
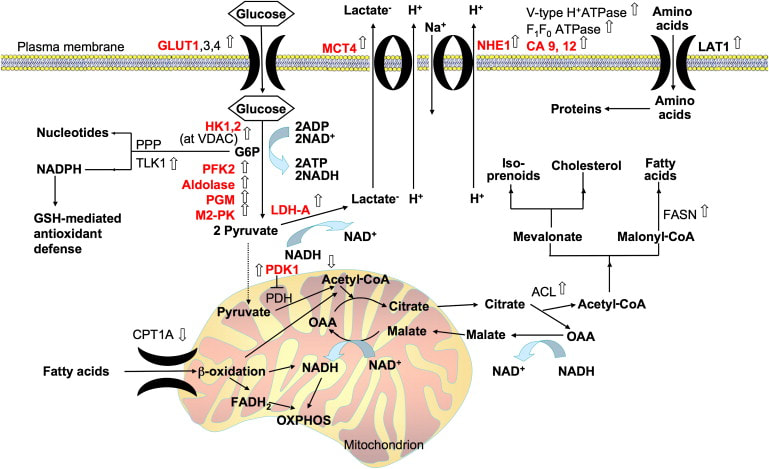
The mitochondria is a eukaryotic organelle critical in the biochemical generation of adenosine triphosphate (ATP) from carbohydrate sources during cellular respiration. ATP is the primary energy carrier for all living organisms and facilitates numerous cellular processes including signaling and differentiation. The electron transport chain (ETC) of the mitochondria is central towards ATP production, forming roughly 34 ATP molecules in a process called oxidative phosphorylation (OXPHOS). OXPHOS broadly involves four ETC proteins (Complexes I-IV) that create a proton gradient across the mitochondrial membrane, and the ATP synthase enzyme which uses this gradient to catalyze ATP formation. These metabolic processes in the mitochondria are key regulators of cell growth, and perturbation at the molecular or genetic level can lead to tumor formation and oncogenesis.
Current research suggests altered behavior of mitochondrial bioenergetics and metabolism is common to cancer cells, allowing them to proliferate even during chemotherapeutic and targeted cancer treatments (Ghosh et al, 2018). Metabolic reprogramming, the rewiring of intracellular metabolic pathways, is an emerging hallmark of tumor biology and a potential therapeutic target for specific cancers (Molina et al, 2018).
Until recently the conventionally held theory on metabolic reprogramming, the Warburg effect, proposed that tumor cells rely more upon the production of cellular building blocks in glycolytic pathways over ATP production through OXPHOS activity for survival. Contrary to the Warburg effect, a recent body of evidence suggests some advanced cancers upregulate their reliance on OXPHOS (Ghosh et al, 2018). In particular, the reprogramming of OXPHOS activity has been experimentally validated in endometrial carcinomas, pancreatic ductal adenocarcinoma, small-cell neuroendocrine prostate cancer, and other cancer phenotypes (Ashton et al, 2018).
Therapeutic strategies of inhibiting the ETC and OXPHOS at the molecular and genetic level have gained support in recent years (Greene et al, 2022). Cancer types vary in prognosis, severity, and their molecular and cellular mechanisms; therefore, the metabolic reprogramming issue for a specific type of cancer does not have a mainstream solution. Instead, scientists can gauge the cancer’s aggressiveness and treatability with biomarkers - a measurement that captures what happens in the cell at any given moment. A prominent biomarker in cancer metabolics is peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α), which functions as a master regulator of mitochondrial function (Boost & Kominski, 2019). PGC-1α is critical in mitochondrial biogenesis - activating downstream transcription factors which control expression of mitochondrial proteins - fatty acid oxidation in response to physiological stimuli, and the regulation of glucose metabolism. Expression levels of biomarkers like PGC-1α, along with many other considerations, can provide a framework for developing an informed strategy for the treatment of a given cancer.
Leading therapeutic strategies for high-OXPHOS cancer phenotypes typically target the ETC complexes that are responsible for OXPHOS. Inhibiting an upstream complex (Complex I or II) would theoretically eliminate the proton gradient essential for ATP production, thereby depriving tumors of the energy that would otherwise allow them to grow. All novel (new) therapeutics for these cancer types are in Phase I of development, which fixates on the molecule’s pharmacology, proper dosage, method of administration, and other similar metrics (U.S. Food and Drug Administration). Encouraging novel therapeutics that inhibit Complex I of the ETC include IACS-010,759 to treat acute myeloid leukemia (Molina et al, 2018) and BAY 87−2243 to treat non-small-cell lung carcinoma (NSCLC) and melanoma (Ellinghaus et al, 2013). Other novel therapeutics utilize other mechanisms to inhibit OXPHOS, for example VLX600 targets colon cancer variants through iron-chelation (removal) in the ETC (Fryknäs et al, 2016). There are many other novel therapeutics with niche mechanisms of action that target niche cancer phenotypes; however, it is important to take a realistic approach to even the most promising therapeutics in early development.
Directing a novel cancer therapeutic to regulatory approval is an arduous procedure of 10-15 years (Derep, 2022) with a meager 3.4% success rate (Berezow, 2020). Rather than starting from scratch, leveraging the biological properties of previous molecules for cancer treatment represents a viable industrial strategy. Previous candidates for anti-diabetic biguanide molecules have been repurposed in clinical trials as cancer preventative agents. Metformin is one of the most widely prescribed diabetes drugs worldwide; however, epidemiological studies also showed it has a significant anti-cancer effect, with a relative reduction risk of approximately 31% (Desensi et al, 2010). Metformin primarily targets complex I of the ETC, and standard clinical doses have shown to activate transcription of multiple mitochondrial metabolic pathways (Lord et al, 2018). Studies suggest Metformin helps inhibit tumor cell proliferation by disrupting their high energy demands via decreased glucose oxidation (Fendt et al, 2013). Further RNA-sequencing has demonstrated Metformin also acts at the transcriptome level, with the degree of change in expression of a mitochondrial OXPHOS gene correlated with changes in a well-validated transcriptomic proliferation signature in breast cancer (Lord et al, 2018). Currently, there are over 390 active trials on Metformin for a wide spectrum of cancers and other diseases.
Another biguanide therapeutic, Phenformin, is being considered for cancer therapy. Similar to Metformin, Phenformin is a biguanide molecule that targets complex I of the ETC. However, Phenformin is more potent and exhibits greater lipophilicity from its larger phenylethyl side chain compared to Metformin. This property enhances Phenoformin’s ability to cross mitochondrial lipid membrane bilayers and allows the molecule to more easily target the ETC, providing an advantage in the context of cancer therapy (Vial et al, 2019). Consistent with these findings, tumor cells have shown susceptibility to Phenformin in preclinical studies on pancreatic cancer (Rajeshkumar, 2017) and NSCLC (Shackelford et al, 2019) in mice. As of 2022, melanoma (skin cancer) patients are being treated with Phenformin in Phase 1 of clinical trials. Researchers from Memorial Sloan-Kettering Centers in New York and the Massachusetts General Hospital are evaluating the safety of combining Phenformin with standard melanoma treatments (Clinical Trial Study Number NCT03026517).
Metofrmin and Phenformin represent some of the most pragmatic therapeutics for the treatment of high-OXPHOS cancer phenotypes. However, rigorous experimentation on even the most promising therapeutics can unveil weaknesses such as side effects, lack of effectiveness in humans, and pharmo-kinetic/dynamic challenges. Thus when therapeutics are conceptually valid or functional in model organisms, it is essential to reflect on all the obstacles a therapeutic must overcome and take a reserved approach for dubbing a molecule as the next “cure” for a cancer or another disease.
Current research suggests altered behavior of mitochondrial bioenergetics and metabolism is common to cancer cells, allowing them to proliferate even during chemotherapeutic and targeted cancer treatments (Ghosh et al, 2018). Metabolic reprogramming, the rewiring of intracellular metabolic pathways, is an emerging hallmark of tumor biology and a potential therapeutic target for specific cancers (Molina et al, 2018).
Until recently the conventionally held theory on metabolic reprogramming, the Warburg effect, proposed that tumor cells rely more upon the production of cellular building blocks in glycolytic pathways over ATP production through OXPHOS activity for survival. Contrary to the Warburg effect, a recent body of evidence suggests some advanced cancers upregulate their reliance on OXPHOS (Ghosh et al, 2018). In particular, the reprogramming of OXPHOS activity has been experimentally validated in endometrial carcinomas, pancreatic ductal adenocarcinoma, small-cell neuroendocrine prostate cancer, and other cancer phenotypes (Ashton et al, 2018).
Therapeutic strategies of inhibiting the ETC and OXPHOS at the molecular and genetic level have gained support in recent years (Greene et al, 2022). Cancer types vary in prognosis, severity, and their molecular and cellular mechanisms; therefore, the metabolic reprogramming issue for a specific type of cancer does not have a mainstream solution. Instead, scientists can gauge the cancer’s aggressiveness and treatability with biomarkers - a measurement that captures what happens in the cell at any given moment. A prominent biomarker in cancer metabolics is peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α), which functions as a master regulator of mitochondrial function (Boost & Kominski, 2019). PGC-1α is critical in mitochondrial biogenesis - activating downstream transcription factors which control expression of mitochondrial proteins - fatty acid oxidation in response to physiological stimuli, and the regulation of glucose metabolism. Expression levels of biomarkers like PGC-1α, along with many other considerations, can provide a framework for developing an informed strategy for the treatment of a given cancer.
Leading therapeutic strategies for high-OXPHOS cancer phenotypes typically target the ETC complexes that are responsible for OXPHOS. Inhibiting an upstream complex (Complex I or II) would theoretically eliminate the proton gradient essential for ATP production, thereby depriving tumors of the energy that would otherwise allow them to grow. All novel (new) therapeutics for these cancer types are in Phase I of development, which fixates on the molecule’s pharmacology, proper dosage, method of administration, and other similar metrics (U.S. Food and Drug Administration). Encouraging novel therapeutics that inhibit Complex I of the ETC include IACS-010,759 to treat acute myeloid leukemia (Molina et al, 2018) and BAY 87−2243 to treat non-small-cell lung carcinoma (NSCLC) and melanoma (Ellinghaus et al, 2013). Other novel therapeutics utilize other mechanisms to inhibit OXPHOS, for example VLX600 targets colon cancer variants through iron-chelation (removal) in the ETC (Fryknäs et al, 2016). There are many other novel therapeutics with niche mechanisms of action that target niche cancer phenotypes; however, it is important to take a realistic approach to even the most promising therapeutics in early development.
Directing a novel cancer therapeutic to regulatory approval is an arduous procedure of 10-15 years (Derep, 2022) with a meager 3.4% success rate (Berezow, 2020). Rather than starting from scratch, leveraging the biological properties of previous molecules for cancer treatment represents a viable industrial strategy. Previous candidates for anti-diabetic biguanide molecules have been repurposed in clinical trials as cancer preventative agents. Metformin is one of the most widely prescribed diabetes drugs worldwide; however, epidemiological studies also showed it has a significant anti-cancer effect, with a relative reduction risk of approximately 31% (Desensi et al, 2010). Metformin primarily targets complex I of the ETC, and standard clinical doses have shown to activate transcription of multiple mitochondrial metabolic pathways (Lord et al, 2018). Studies suggest Metformin helps inhibit tumor cell proliferation by disrupting their high energy demands via decreased glucose oxidation (Fendt et al, 2013). Further RNA-sequencing has demonstrated Metformin also acts at the transcriptome level, with the degree of change in expression of a mitochondrial OXPHOS gene correlated with changes in a well-validated transcriptomic proliferation signature in breast cancer (Lord et al, 2018). Currently, there are over 390 active trials on Metformin for a wide spectrum of cancers and other diseases.
Another biguanide therapeutic, Phenformin, is being considered for cancer therapy. Similar to Metformin, Phenformin is a biguanide molecule that targets complex I of the ETC. However, Phenformin is more potent and exhibits greater lipophilicity from its larger phenylethyl side chain compared to Metformin. This property enhances Phenoformin’s ability to cross mitochondrial lipid membrane bilayers and allows the molecule to more easily target the ETC, providing an advantage in the context of cancer therapy (Vial et al, 2019). Consistent with these findings, tumor cells have shown susceptibility to Phenformin in preclinical studies on pancreatic cancer (Rajeshkumar, 2017) and NSCLC (Shackelford et al, 2019) in mice. As of 2022, melanoma (skin cancer) patients are being treated with Phenformin in Phase 1 of clinical trials. Researchers from Memorial Sloan-Kettering Centers in New York and the Massachusetts General Hospital are evaluating the safety of combining Phenformin with standard melanoma treatments (Clinical Trial Study Number NCT03026517).
Metofrmin and Phenformin represent some of the most pragmatic therapeutics for the treatment of high-OXPHOS cancer phenotypes. However, rigorous experimentation on even the most promising therapeutics can unveil weaknesses such as side effects, lack of effectiveness in humans, and pharmo-kinetic/dynamic challenges. Thus when therapeutics are conceptually valid or functional in model organisms, it is essential to reflect on all the obstacles a therapeutic must overcome and take a reserved approach for dubbing a molecule as the next “cure” for a cancer or another disease.
References
Ashton TM, McKenna WG, Kunz-Schughart LA, Higgins GS. 2018. Oxidative Phosphorylation as an Emerging Target in Cancer Therapy. Clinical Cancer Research. 2842
Berezow, Alex. “Clinical Trial Success Rates by Phase and Therapeutic Area.” American Council on Science and Health, 12 June 2020, https://www.acsh.org/news/2020/06/11/clinical-trial-success-rates-phase-and-therapeutic-area-14845#:~:text=As%20shown%2C%20the%20overall%20probability%20of%20success%20for,number%20masks%20a%20wide%20variation%20by%20therapeutic%20area.
Bost F, Kaminski L. 2019. The metabolic modulator PGC-1α in cancer. American Journal of Cancer Research. 198-211.
Commissioner, Office of the. “Step 1: Discovery and Development.” U.S. Food and Drug Administration, FDA, https://www.fda.gov/patients/drug-development-process/step-1-discovery-and-development.
Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, Gandini S. 2010. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prevention Research. 1451-61.
Derep, Maxime. What's the Average Time to Bring a Drug to Market in 2022? N-SIDE, 11 May 2022, https://lifesciences.n-side.com/blog/what-is-the-average-time-to-bring-a-drug-to-market-in-2022.
Ellinghaus P, Heisler I, Unterschemmann K, Haerter M, Beck H, Greschat S, Ehrmann A, Summer H, Flamme I, Oehme F, Thierauch K, Michels M, Hess-Stumpp H, Ziegelbauer K. 2013. BAY 87-2243, a highly potent and selective inhibitor of hypoxia-induced gene activation has antitumor activities by inhibition of mitochondrial complex I. Cancer Medicine. 611-24.
Fendt SM, Bell EL, Keibler MA, Davidson SM, Wirth GJ, Fiske B, Mayers JR, Schwab M, Bellinger G, Csibi A, Patnaik A, Blouin MJ, Cantley LC, Guarente L, Blenis J, Pollak MN, Olumi AF, Vander Heiden MG, Stephanopoulos G. 2013. Metformin decreases glucose oxidation and increases the dependency of prostate cancer cells on reductive glutamine metabolism. Cancer Research. 4429-38.
Ghosh P, Vidal C, Dey S, Zhang L. 2020. Mitochondria Targeting as an Effective Strategy for Cancer Therapy. International Journal of Molecular Sciences. 3363.
Greene J, Segaran A, Lord S. 2022. Targeting OXPHOS and the electron transport chain in cancer; Molecular and therapeutic implications. Seminars in Cancer Biology. 851-859.
Lord SR, Cheng WC, Liu D, Gaude E, Haider S, Metcalf T, Patel N, Teoh EJ, Gleeson F, Bradley K, Wigfield S, Zois C, McGowan DR, Ah-See ML, Thompson AM, Sharma A, Bidaut L, Pollak M, Roy PG, Karpe F, James T, English R, Adams RF, Campo L, Ayers L, Snell C, Roxanis I, Frezza C, Fenwick JD, Buffa FM, Harris AL. 2018. Integrated Pharmacodynamic Analysis Identifies Two Metabolic Adaption Pathways to Metformin in Breast Cancer. Cell Metabolism. 679-688.
Molina JR, Sun Y, Protopopova M, Gera S, Bandi M, Bristow C, McAfoos T, Morlacchi P, Ackroyd J, Agip AA, Al-Atrash G, Asara J, Bardenhagen J, Carrillo CC, Carroll C, Chang E, Ciurea S, Cross JB, Czako B, Deem A, Daver N, de Groot JF, Dong JW, Feng N, Gao G, Gay J, Do MG, Greer J, Giuliani V, Han J, Han L, Henry VK, Hirst J, Huang S, Jiang Y, Kang Z, Khor T, Konoplev S, Lin YH, Liu G, Lodi A, Lofton T, Ma H, Mahendra M, Matre P, Mullinax R, Peoples M, Petrocchi A, Rodriguez-Canale J, Serreli R, Shi T, Smith M, Tabe Y, Theroff J, Tiziani S, Xu Q, Zhang Q, Muller F, DePinho RA, Toniatti C, Draetta GF, Heffernan TP, Konopleva M, Jones P, Di Francesco ME, Marszalek JR. 2018. An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nature Medicine. 1036-1046.
Rajeshkumar NV, Yabuuchi S, Pai SG, De Oliveira E, Kamphorst JJ, Rabinowitz JD, Tejero H, Al-Shahrour F, Hidalgo M, Maitra A, Dang CV. Treatment of Pancreatic Cancer Patient-Derived Xenograft Panel with Metabolic Inhibitors Reveals Efficacy of Phenformin. 2017. Clinical Cancer Research. 5639-5647.
Shackelford DB, Abt E, Gerken L, Vasquez DS, Seki A, Leblanc M, Wei L, Fishbein MC, Czernin J, Mischel PS, Shaw RJ. 2013. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 143-58.
Vial G, Detaille D, Guigas B. 2019. Role of Mitochondria in the Mechanism(s) of Action of Metformin. Frontiers in Endocrinology (Lausanne). 1-8.