Suchita Kumar, Class of 2021
Notch signaling is an evolutionarily-conserved signaling mechanism involved in several stages of development. Although the core signaling pathway has a simple molecular design, Notch signaling is responsible for several distinct cell-fate decisions including proliferation, differentiation, border formation, and apoptosis. Since cell-fate decisions are critical to embryonic development and postnatal tissue regeneration, misregulation or loss-of-function mutations in genes related to Notch signaling can lead to diseases in various tissues and organs (Andersson, 2011). In humans, mutations in the Notch signaling pathway have been found to cause Alagille syndrome, Spondylocostal dysostosis, and Hajdu-Cheney syndrome (Harper, 2003).
Notch signaling can be traced back to the emergence of metazoa, which is the monophyletic group of multicellular organisms thought to have evolved 1,300-600 million years ago during the pre-Ediacaran period (Muller, 2003). The development of multicellularity required the simultaneous development of intercellular communication. With further evolution of eumetazoa, which includes the cnidaria and bilateria with differentiated tissues, there was a need for more complex intercellular signaling to coordinate the development of distinct tissues and organs (Dewel, 2000). In order to establish body axes and choreograph morphogenesis during development, metazoans rely on only seven core signaling pathways, one of which is Notch (Gazave, 2009).
Notch signaling can be traced back to the emergence of metazoa, which is the monophyletic group of multicellular organisms thought to have evolved 1,300-600 million years ago during the pre-Ediacaran period (Muller, 2003). The development of multicellularity required the simultaneous development of intercellular communication. With further evolution of eumetazoa, which includes the cnidaria and bilateria with differentiated tissues, there was a need for more complex intercellular signaling to coordinate the development of distinct tissues and organs (Dewel, 2000). In order to establish body axes and choreograph morphogenesis during development, metazoans rely on only seven core signaling pathways, one of which is Notch (Gazave, 2009).
Figure 1
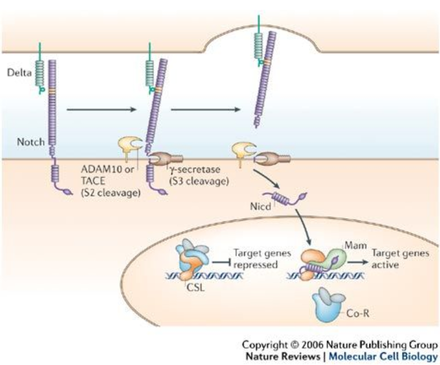
The core Notch pathway, as seen in Figure 1, consists of the transmembrane Notch receptor and the Delta ligand, both of which have an extracellular domain. Binding of the extracellular domains of the Delta ligand and Notch receptor leads to two cleavage events. The first cleavage event is catalyzed by ADAM, a metalloprotease that cleaves the extracellular domain of Notch, and the second is catalyzed by Ɣ-secretase, an enzyme complex that releases the Notch intracellular domain (Nicd). The translocation of Nicd to the nucleus and its further association with the CSL protein and co-activator Mastermind (Mam) is responsible for promoting transcription (Bray, 2006).
Unlike other signaling pathways, there is no amplification of the signal, causing each activated Notch receptor to release one Nicd. This stoichiometric relationship between signaling input and output makes Notch signaling extremely sensitive to dosage, which explains why haploinsufficiency or the presence of extra copies of Notch-related genes leads to aberrant phenotypes (Andersson, 2011). In humans, haploinsufficiency of JAG1, one of the ligands that binds to the Notch receptor, causes Alagille syndrome and haploinsufficiency of NOTCH1, one of the four Notch receptors in humans, causes aortic valve disease (Andersson, 2011).
Unlike other signaling pathways, there is no amplification of the signal, causing each activated Notch receptor to release one Nicd. This stoichiometric relationship between signaling input and output makes Notch signaling extremely sensitive to dosage, which explains why haploinsufficiency or the presence of extra copies of Notch-related genes leads to aberrant phenotypes (Andersson, 2011). In humans, haploinsufficiency of JAG1, one of the ligands that binds to the Notch receptor, causes Alagille syndrome and haploinsufficiency of NOTCH1, one of the four Notch receptors in humans, causes aortic valve disease (Andersson, 2011).
Figure 2
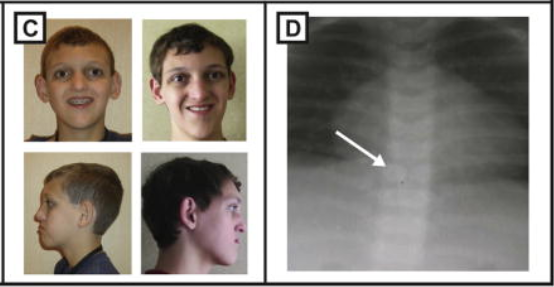
Alagille syndrome (ALGS) is characterized by liver disease, cardiac disease, skeletal anomalies, and renal anomalies. Liver disease resulting from intrahepatic bile duct paucity is the most common phenotype of ALGS, which occurs due to the role of Notch signaling in bile duct formation. Cardiac disease is also observed in ALGS in the form of vascular stenosis (narrowing of arteries and veins) and defects in the atrioventricular septum that divides the left and right chambers of the heart. ALGS also causes skeletal anomalies like butterfly vertebrae as seen in Figure 2, resulting from the vertebral arches failing to fuse, and renal anomalies due to the role of Notch signaling in nephron segmentation (Harper, 2003). These phenotypic aberrations further make evident the importance of Notch signaling in binary cell-fate decisions involving segmentation, fusion and other morphogenesis.
Figure 3
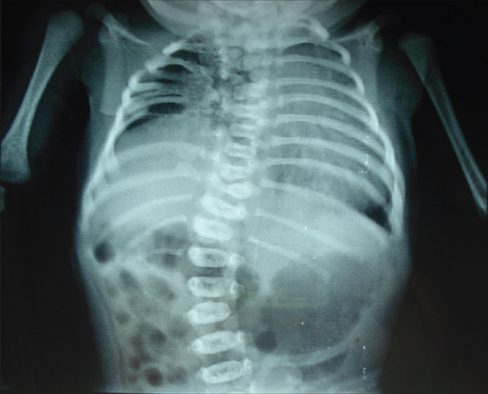
Spondylocostal dysostosis (SCD) and Spondylothoracic dysostosis (STD) are diseases that fall under the category of abnormal vertebral segmentation (AVS), which are characterized by congenital malformations in which vertebrae are fused or altered in shape, position, or size. The cause of these diseases is a mutation in the Delta-like ligand 3 (DLL3), which does not bind to the Notch receptor, but inhibits Notch receptor activity by targeting it for lysosomal degradation. Mutation of DLL3 causes an increase in Notch signaling, preventing proper somitogenesis, which is the formation of the vertebral column, rib cage, skeletal muscle, cartilage, and tendons (Harper, 2003). This leads to the misalignment and fusion of vertebrae and ribs as seen in Figure 3 above.
Hajdu-Cheney syndrome (HCS) is a rare condition characterized by osteoporosis and craniofacial abnormalities. It is the result of mutation of the NOTCH2 receptor, which leads to a positive feedback loop that increases its own signaling. This increase in Notch signaling prevents proper bone formation and metabolism (Harper, 2003). Since HCS commonly presents with a cleft palate, this further emphasises the role played by Notch signaling in establishing bilateral symmetry.
The role of Notch signaling in morphogenesis is still not fully understood as it is simultaneously responsible for the activation of certain cell-fate decision pathways and the termination of others. However, it is evident that due to the importance of Notch signaling in several tissues, especially at the stage of embryonic development, mutation to genes involved in the Notch signaling pathway affects more than a single organ system. On evaluating Alagille syndrome, Spondylocostal dysostosis, and Hajdu-Cheney syndrome, Notch signaling appears to be most critical to hepatic, cardiac, and skeletal development. Studying these conditions and their association with Notch signaling is not only useful in gaining a better understanding of the complex role of Notch signaling in humans, but is also critical to developing treatments for the conditions.
References
1. Andersson, E. R., Sandberg, R., & Lendahl, U. (2011). Notch signaling: Simplicity in design, versatility in function. Development,138(17), 3593-3612. doi:10.1242/dev.063610
2. Gazave, E., Lapébie, P., Richards, G. S., Brunet, F., Ereskovsky, A. V., Degnan, B. M., . . . Renard, E. (2009). Origin and evolution of the Notch signalling pathway: An overview from eukaryotic genomes. BMC Evolutionary Biology,9(1), 249. doi:10.1186/1471-2148-9-249
3. Muller, W. E. (2003). The Origin of Metazoan Complexity: Porifera as Integrated Animals. Integrative and Comparative Biology,43(1), 3-10. doi:10.1093/icb/43.1.3
4. Dewel, R. A. (2000). Colonial origin for Eumetazoa: Major morphological transitions and the origin of bilaterian complexity. Journal of Morphology,243(1), 35-74. doi:10.1002/(sici)1097-4687(200001)243:13.0.co;2-#
5. Bray, S. J. (2006). Notch signalling: A simple pathway becomes complex. Nature Reviews Molecular Cell Biology,7(9), 678-689. doi:10.1038/nrm2009
6. Harper, J., Yuan, J., Tan, J., Visan, I., & Guidos, C. (2003). Notch signaling in development and disease. Clinical Genetics,64(6), 461-472. doi:10.1046/j.1399-0004.2003.00194.x
7. Thakur, N., & Rai, N. (2016). Case series of spondylocostal dysostosis and associated congenital malformations. Journal of Clinical Neonatology,5(3), 209. doi:10.4103/2249-4847.191271
Hajdu-Cheney syndrome (HCS) is a rare condition characterized by osteoporosis and craniofacial abnormalities. It is the result of mutation of the NOTCH2 receptor, which leads to a positive feedback loop that increases its own signaling. This increase in Notch signaling prevents proper bone formation and metabolism (Harper, 2003). Since HCS commonly presents with a cleft palate, this further emphasises the role played by Notch signaling in establishing bilateral symmetry.
The role of Notch signaling in morphogenesis is still not fully understood as it is simultaneously responsible for the activation of certain cell-fate decision pathways and the termination of others. However, it is evident that due to the importance of Notch signaling in several tissues, especially at the stage of embryonic development, mutation to genes involved in the Notch signaling pathway affects more than a single organ system. On evaluating Alagille syndrome, Spondylocostal dysostosis, and Hajdu-Cheney syndrome, Notch signaling appears to be most critical to hepatic, cardiac, and skeletal development. Studying these conditions and their association with Notch signaling is not only useful in gaining a better understanding of the complex role of Notch signaling in humans, but is also critical to developing treatments for the conditions.
References
1. Andersson, E. R., Sandberg, R., & Lendahl, U. (2011). Notch signaling: Simplicity in design, versatility in function. Development,138(17), 3593-3612. doi:10.1242/dev.063610
2. Gazave, E., Lapébie, P., Richards, G. S., Brunet, F., Ereskovsky, A. V., Degnan, B. M., . . . Renard, E. (2009). Origin and evolution of the Notch signalling pathway: An overview from eukaryotic genomes. BMC Evolutionary Biology,9(1), 249. doi:10.1186/1471-2148-9-249
3. Muller, W. E. (2003). The Origin of Metazoan Complexity: Porifera as Integrated Animals. Integrative and Comparative Biology,43(1), 3-10. doi:10.1093/icb/43.1.3
4. Dewel, R. A. (2000). Colonial origin for Eumetazoa: Major morphological transitions and the origin of bilaterian complexity. Journal of Morphology,243(1), 35-74. doi:10.1002/(sici)1097-4687(200001)243:13.0.co;2-#
5. Bray, S. J. (2006). Notch signalling: A simple pathway becomes complex. Nature Reviews Molecular Cell Biology,7(9), 678-689. doi:10.1038/nrm2009
6. Harper, J., Yuan, J., Tan, J., Visan, I., & Guidos, C. (2003). Notch signaling in development and disease. Clinical Genetics,64(6), 461-472. doi:10.1046/j.1399-0004.2003.00194.x
7. Thakur, N., & Rai, N. (2016). Case series of spondylocostal dysostosis and associated congenital malformations. Journal of Clinical Neonatology,5(3), 209. doi:10.4103/2249-4847.191271